Process and Methodology
From Setup to Success: An Overview
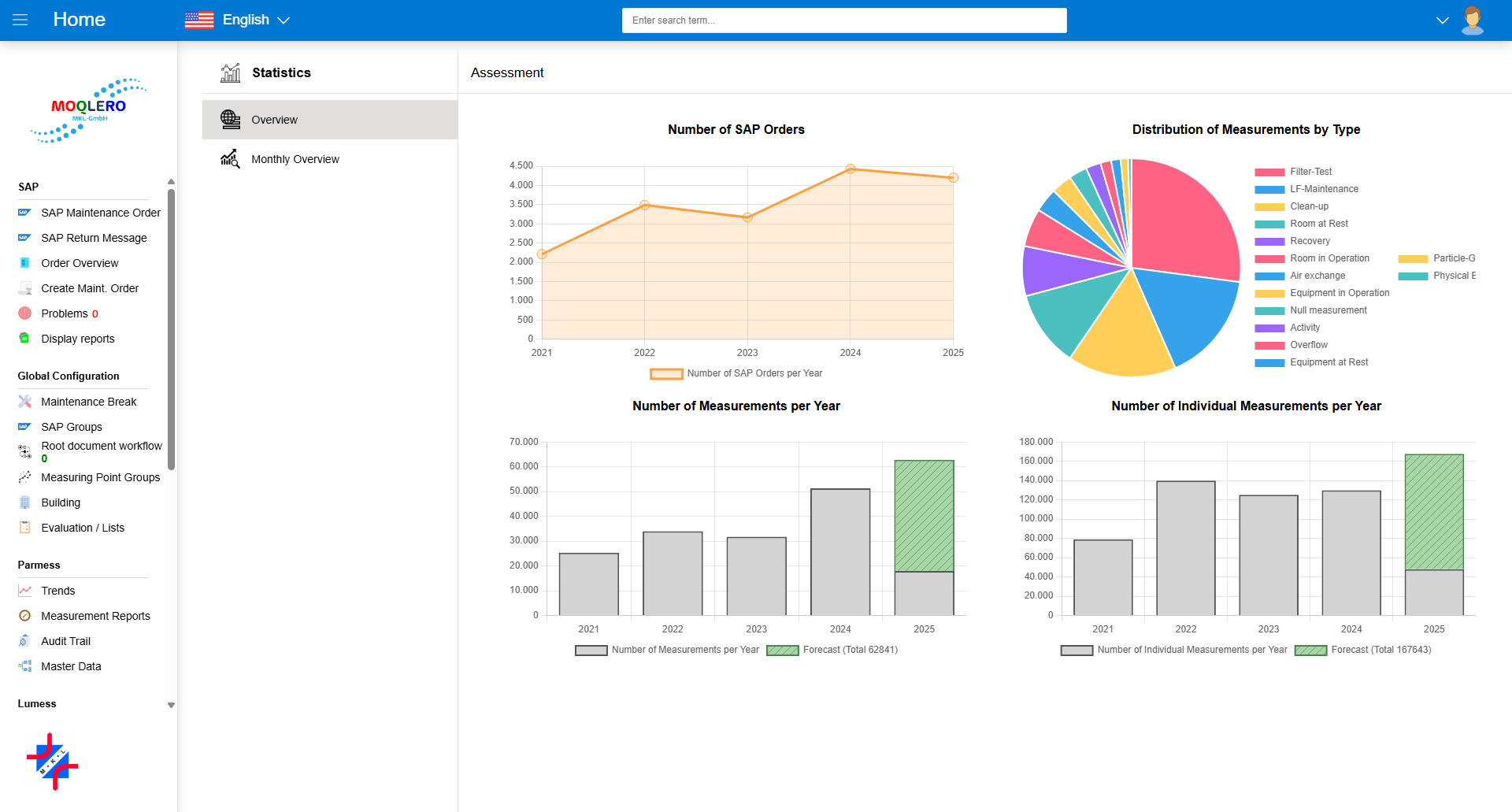
Not Another Continuous Monitoring Tool.
Continuous, sensor-driven monitoring solutions are excessive for many clean environments, both in terms of cost and complexity.
Recognizing this gap, Moqlero is engineered to meet the exacting demands for automation in discontinuous monitoring environments.
It digitizes manual, scheduled, and on demand ad-hoc measurements, avoiding data overload and instead focusing on timely, and targeted measurements.
Its core area of application is executing scheduled and event based monitoring, classification and recertification of cleanroom environments.
Automated control of particle counters and evaluation of measurement results are naturally included.